Quick Menu
Pharmaceutical Solutions
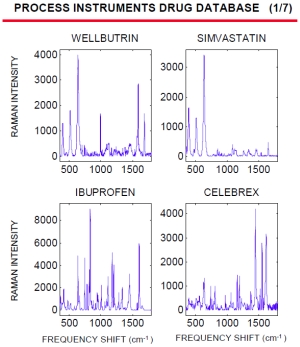
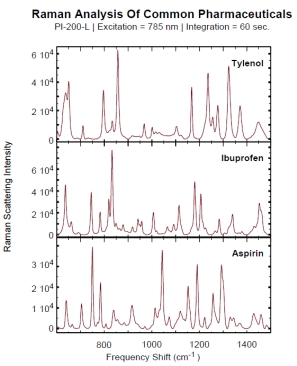
Raman spectroscopy can very quickly identify and characterize the active pharmaceutical ingredient inside a pill. Below is a short summary of our current drug database. Spectra for the different drugs are displayed to the right (click on picture to open a full detailed pdf version). Below is a summary of our current drug database.
Due to the unique signature each drug generates, a spectral drug database can be used to detect counterfeit drugs or as a method of validating that the correct dosage/API is in the proper vial.
| WELLBUTRIN | SIMVASTATIN | IBUPROFEN | CELEBREX |
| VERAPAMIL | AMITRIPTYLINE HCL | LISINOPRIL | RANITIDINE (Zantac) |
| TIAZAC | OLANZAPINE | FUROSEMIDE | LORATADINE |
| CALCIUM / VITAMIN D | ETODOLAC (Lodine) | VITAMIN B1 | ISOSORBIDE DINITRATE |
| ASPIRIN | BUSPAR (Buspirone) | SINGULAIR | DIGOXIN |
| MAG 64 (MgCl) | GABAPENTIN | ACIPHEX | PLENDIL (Felodipine) |
| COUMADIN (Warfarin) | OMEPRAZOLE | BENADRYL | DOXAZOSIN MESYLATE |
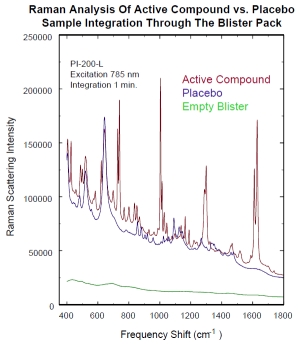
Active Compound versus a Placebo
Raman analysis of an API compound vs. Placebo through the blister pack. The spectra are clearly different in chemistry and no Raman signal is observed from an empty blister pack. Raman analysis can quickly (< 40 ms) identify an active compound through the blister pack.